Examples Of Buffers In Biological Systems . It takes only seconds for the chemical. Web the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. Web biological buffers are compounds that help the body maintain a ph around 7.4. Web biological buffers are organic substances that help regulate the ph level in organisms. They act by neutralizing excess. Web buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical. Web the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. Web the buffer systems in the human body are extremely efficient, and different systems work at different rates. Web you should be aware that buffers play a critical role in almost all biochemical systems.
from www.youtube.com
Web buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical. Web the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. Web you should be aware that buffers play a critical role in almost all biochemical systems. They act by neutralizing excess. Web the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. It takes only seconds for the chemical. Web the buffer systems in the human body are extremely efficient, and different systems work at different rates. Web biological buffers are compounds that help the body maintain a ph around 7.4. Web biological buffers are organic substances that help regulate the ph level in organisms.
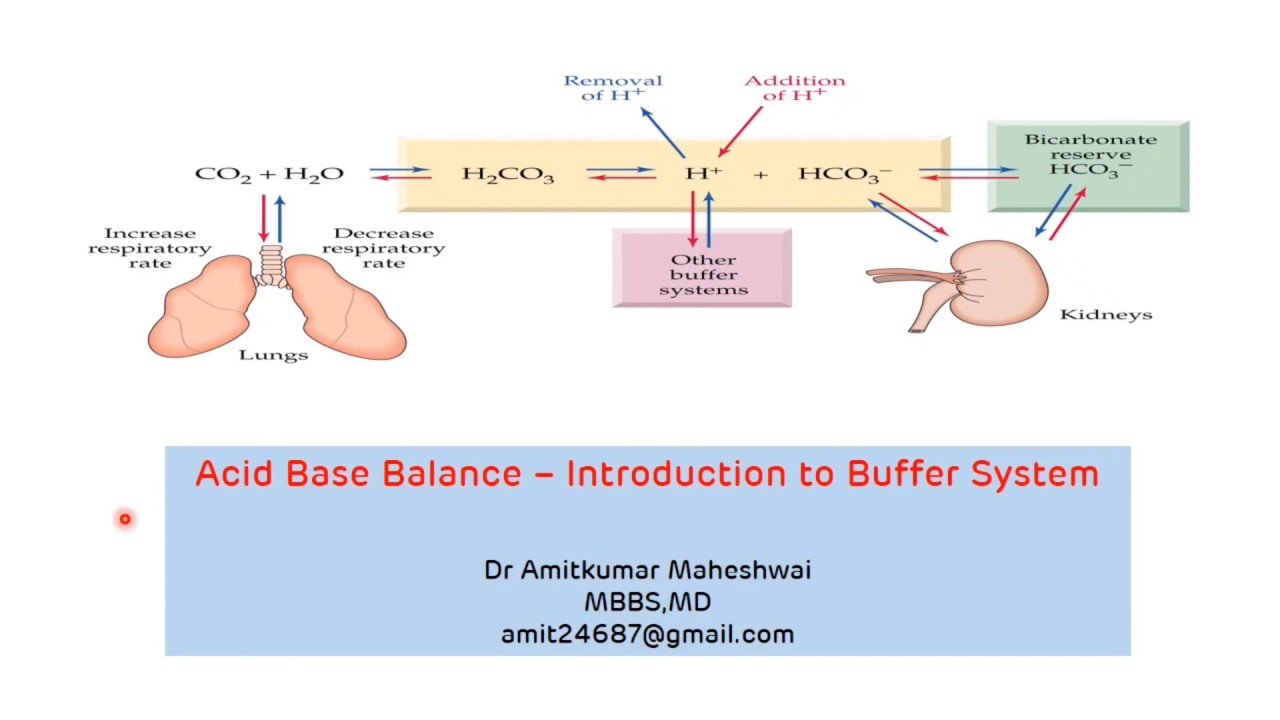
Introduction to Buffer System Regulation of pH Acid Base Balance
Examples Of Buffers In Biological Systems It takes only seconds for the chemical. Web you should be aware that buffers play a critical role in almost all biochemical systems. Web the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. Web the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. Web biological buffers are organic substances that help regulate the ph level in organisms. They act by neutralizing excess. It takes only seconds for the chemical. Web buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical. Web the buffer systems in the human body are extremely efficient, and different systems work at different rates. Web biological buffers are compounds that help the body maintain a ph around 7.4.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Examples Of Buffers In Biological Systems They act by neutralizing excess. Web the buffer systems in the human body are extremely efficient, and different systems work at different rates. Web the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. Web the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood. Examples Of Buffers In Biological Systems.
From www.youtube.com
Buffers and pH Biology YouTube Examples Of Buffers In Biological Systems Web you should be aware that buffers play a critical role in almost all biochemical systems. Web the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. It takes only seconds for the chemical. They act by neutralizing excess. Web buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise. Examples Of Buffers In Biological Systems.
From ceaaclpe.blob.core.windows.net
Buffer In Biology Example at Richard Durbin blog Examples Of Buffers In Biological Systems Web buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical. Web the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. It takes only seconds for the chemical. Web the purpose of a buffer in a biological system is to maintain intracellular and extracellular. Examples Of Buffers In Biological Systems.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Examples Of Buffers In Biological Systems It takes only seconds for the chemical. Web the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. Web the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. Web biological buffers are organic substances that help regulate the ph level in organisms. Web. Examples Of Buffers In Biological Systems.
From www.slideshare.net
Buffers in biological systems Examples Of Buffers In Biological Systems Web buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical. Web the buffer systems in the human body are extremely efficient, and different systems work at different rates. It takes only seconds for the chemical. Web the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls. Examples Of Buffers In Biological Systems.
From www.youtube.com
buffer isotonic solution buffer capacity buffers in pharmaceutical Examples Of Buffers In Biological Systems It takes only seconds for the chemical. Web biological buffers are organic substances that help regulate the ph level in organisms. They act by neutralizing excess. Web buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical. Web the purpose of a buffer in a biological system is to maintain intracellular. Examples Of Buffers In Biological Systems.
From www.slideserve.com
PPT UNIT IV PowerPoint Presentation, free download ID4510549 Examples Of Buffers In Biological Systems They act by neutralizing excess. Web the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. Web the buffer systems in the human body are extremely efficient, and different systems work at different rates. It takes only seconds for the chemical. Web buffers play an immense role in biological systems where maintaining constant internal. Examples Of Buffers In Biological Systems.
From ceaaclpe.blob.core.windows.net
Buffer In Biology Example at Richard Durbin blog Examples Of Buffers In Biological Systems Web biological buffers are organic substances that help regulate the ph level in organisms. They act by neutralizing excess. Web buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical. It takes only seconds for the chemical. Web the most relevant systems for biology are the carbonic acid/carbonate buffering system, which. Examples Of Buffers In Biological Systems.
From www.leinco.com
Biological Buffers And pH Range Leinco Technologies Examples Of Buffers In Biological Systems Web buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical. It takes only seconds for the chemical. Web the buffer systems in the human body are extremely efficient, and different systems work at different rates. Web you should be aware that buffers play a critical role in almost all biochemical. Examples Of Buffers In Biological Systems.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Examples Of Buffers In Biological Systems Web the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. Web buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical. They act by neutralizing excess. Web biological buffers are organic substances that help regulate the ph level in organisms. Web biological buffers are. Examples Of Buffers In Biological Systems.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Examples Of Buffers In Biological Systems Web the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. Web biological buffers are organic substances that help regulate the ph level in organisms. Web the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. They act by neutralizing excess. Web you should. Examples Of Buffers In Biological Systems.
From www.youtube.com
Buffer Capacity 03 Buffers in pharmaceutical and biological systems Examples Of Buffers In Biological Systems It takes only seconds for the chemical. Web the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. Web biological buffers are compounds that help the body maintain a ph around 7.4. Web the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. Web. Examples Of Buffers In Biological Systems.
From www.slideserve.com
PPT UNIT IV PowerPoint Presentation, free download ID4510549 Examples Of Buffers In Biological Systems Web biological buffers are compounds that help the body maintain a ph around 7.4. Web biological buffers are organic substances that help regulate the ph level in organisms. Web the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. Web the buffer systems in the human body are extremely efficient, and different systems work. Examples Of Buffers In Biological Systems.
From www.slideshare.net
Buffer system Examples Of Buffers In Biological Systems Web the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. Web biological buffers are compounds that help the body maintain a ph around 7.4. Web buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical. Web the most relevant systems. Examples Of Buffers In Biological Systems.
From exonxjimh.blob.core.windows.net
Common Buffers Used In Biochemistry at Betty Beckles blog Examples Of Buffers In Biological Systems Web the buffer systems in the human body are extremely efficient, and different systems work at different rates. Web buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical. Web biological buffers are compounds that help the body maintain a ph around 7.4. Web biological buffers are organic substances that help. Examples Of Buffers In Biological Systems.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Examples Of Buffers In Biological Systems Web buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical. They act by neutralizing excess. Web biological buffers are compounds that help the body maintain a ph around 7.4. Web biological buffers are organic substances that help regulate the ph level in organisms. Web the purpose of a buffer in. Examples Of Buffers In Biological Systems.
From www.slideserve.com
PPT UNIT IV PowerPoint Presentation, free download ID4510549 Examples Of Buffers In Biological Systems It takes only seconds for the chemical. They act by neutralizing excess. Web the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. Web you should be aware that buffers play a critical role in almost all biochemical systems. Web biological buffers are organic substances that help regulate the ph level in organisms. Web. Examples Of Buffers In Biological Systems.
From ceaaclpe.blob.core.windows.net
Buffer In Biology Example at Richard Durbin blog Examples Of Buffers In Biological Systems It takes only seconds for the chemical. Web the buffer systems in the human body are extremely efficient, and different systems work at different rates. Web buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical. Web biological buffers are compounds that help the body maintain a ph around 7.4. Web. Examples Of Buffers In Biological Systems.